Transformation of Zidovudine during Chlorination Process: Kinetics
Transformation OF ZIDOVUDINE DURING CHLORINATION PROCESS: KINECTICS
Abstraction
Progresss in H2O quality Analysis indicate that antibiotics are emerging pollutants due to their uninterrupted input and continuity in the aquatic ecosystem even at low concentrations. Research workers across the universe reveal that in environmental matrices, bespeaking conventional intervention methods are inefficient in their remotion.To prevent this taint ; several procedures to degrade/ take antibiotics have been studied. Present study addresses the current province of cognition refering the debasement and remotion procedures applied to a specific category of antibiotics. Numerous inorganic and organic micro pollutants can undergo reactions with Cl. Chlorine responsiveness is limited to particular sites ( chiefly aminoalkanes, reduced sulfur medieties or activated aromatic system ) and is normally observed during chlorination procedures. HOCl is the major reactive Cl species during chlorination processes.To better understand /predict Cl reaction with micro pollutants, the dynamicss and mechanistic information on Cl responsiveness, this Research was taken up.The nowadays survey reveals that debasement degrees are highest at low pH scopes and able to take approximately 50 % under standard trial conditions.
Keywords:Chlorination, Kinetics, pharmaceutical drug, etc.
1: Introduction
Zidovudine ( INN ) or azido-thymidine ( AZT ) is a nucleoside parallel contrary RNA polymerase inhibitor ( NRTI ) , a type of antiretroviral drug[ 13-14 ]. It is a man-made drug with pyrimidine nucleoside parallel active against HIV-1, AIDS and pre- AIDS. The chemical name of Zidovudine is 1- ( 3- azide-2, 3-di deoxy-?-D-ribofuranosyl ) -5-methyl Pyrimidin-2, 4 ( 1H, 3H ) – dione. Zidovudine besides has been referred to as 3?azido-3?-deoxythymidine. It has a molecular expression of C10Hydrogen13Nitrogen5Oxygen4and a molecular weight of 267.24 g/mol. It has the structural expression as shown in Figure No.1. Zidovudine is a white to light brown, odorless, crystalline solid and it is soluble in ethyl alcohol ( 95 % ) , vigilantly soluble in H2O. The drug is magisterially listed in United States of Pharmacopiea[ 11 ]. Numerous systematic methods that have been reported for the rating of Zidovudine in biological fluids or pharmaceutical preparations include UV-Visible Spectrophotometry, High Performance Liquid Chromatography and HPTLC.
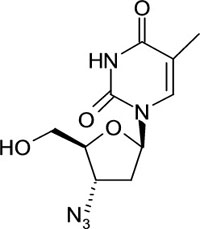
Fig 1: Structure of Zidovudine
Several imbibing H2O beginnings have been contaminated with pesticides, pharmaceuticals and anti microbic agents, every bit good as other potentially harmful chemicals.The destiny for many of these compounds under imbibing H2O intervention conditions is of major concern due to their possible inauspicious wellness effects excite distribution of antibacterial among native bacteriums. A immense part of the entire clinically prescribed antibacterial burden is discharged into municipal waste H2O systems, which represent a chief way for entry of such human usage antibacterial agents into natural aquatic environment[ 9 ].
Chlorination is a chemical procedure normally used in H2O intervention particularly for disinfection. Drinking H2O disinfection normally involves the usage of Cl at one or two points in the intervention procedure, i.e. , for pretreatment ( to bring on a primary disinfection at the beginning of the intervention procedure ) and/or for post-treatment ( to keep a bactericidal remainder in the distribution system ) . Due to their capableness for disinfection ( micro-organisms ) and oxidization ( e.g. gustatory sensation and olfactory property control, riddance of micro pollutants, etc. ) , chemical oxidizers ( i.e. ozone, Cl, Cl dioxide, chloramines, etc. ) are normally used in H2O intervention procedures[ 8 ].
Pharmaceuticals together with antibiotics are present in municipal sewerage, chiefly as a consequence of human elimination. Many active antibiotics are non wholly metabolized during curative usage and therefore travel in sewerage through elimination in an unaffected type. The planned disposal of fresh drugs into the cloaca and veterinary usage besides contribute to the measures of antibiotics originate in sewerage. Discharges from veterinary clinics and overflow from agricultural applications into municipal cloacas are besides possible beginnings of veterinary antibiotics in effluent. The reported degrees of exact antibiotic drugs detected in natural sewerage come into position to differ between states, possibly shimmering mutable recommendation patterns and differences in per-capita H2O usage taking to assorted grades of dilution[ 6 ].
2 Materials and Methodology
All difficult work was made to completion the conductivity of experiments with standard processs as per standard literature referred.
A stock solution of Retrovir was arranged by fade outing suited sum of sample in distilled H2O. A stock solution of HOCl was set by taking an suited volume of 5 % NaOCl in deionised H2O[ 4 ]. The stock solution was so uniformed by iodometry and DPD ( N, N-Diethyl-P-Phenylenediamine ) -FAS ( Ferric Ammonium Sulfate ) titrimetry severally. 0.02M ethanoate ( pH 4 – 5 ) , phosphate ( pH 6 – 8.5 ) , and borate ( pH 9 ) buffers were used to uphold changeless pH through experiments conducted in reagent H2O[ 7 ].
All reagents were of analytical class.
- The Rate of Degradation was assessed i.e ( Kinetic Rates ) by ; a CARY 50 Bio UV-Vis Spectrophotometer ( Varian BV, The Netherlands ) with temperature accountant was instrumental.
- For pH analysis, an Elico pH metre theoretical account LI 120 was used[ 7 ].

Fig 2: CARY 50 Bio UV-Vis Spectro-photometer ( Varian BV, the Netherlands ) with temperature accountant. ( Beginning: Chemistry research lab G.I.T Belgaum)
2.1 Kinetic Measurements
The kinetic measuring were performed under imposter first order status with HOCl was at least 10 fold grinder surplus over ZDV at an invariable ionic strength utilizing 0.02 mol diabetes mellitus-3buffers. The reaction was initiated by blending thermo-statted solutions of FAC and ZDV which besides contains the indispensable volume of buffers. The temperature was systematically maintained at ( 25 ± 0.2 ) oC. The ground of the reaction was followed by supervising lessen in the optical density of ZDV as a function of clip in a 1 cm way length vitreous silica cell of Carry 50 Bio UV-Visible spectro-photometer. The intent of Beer’s jurisprudence of ZDV at ?soap266 nanometer had been confirmed giving ?=10116 diabetes mellitus3mol-1centimeter-1Pseudo first-order rate invariables, k’obs, were evaluated from the secret plans of log ( AT– Angstrom?) versus clip, where ‘A’ refers to absorbance at any clip T and T?is at infinite clip which excludes the optical density of any merchandises of ZDV during the reaction. The first-order secret plans in about all the instances were additive up to 80 % decision of the reaction and ka??obs ( Table 1 )[ 7 ].
Table. 2.1: Consequence of fluctuation of [ HOCl ] and [ ZDV ] on the rate of chlorination of ZDV at 250C. Ionic strength ; 0.02 mol diabetes mellitus-3at 250C
|
pH |
[ HOCl ] ( M ) |
[ ZDV ] ( M ) |
K Obs ( s-1) |
|
3 |
3.22×10-4 |
5×10-5 |
3.0×10-3 |
|
6.45×10-4 |
5×10-5 |
3.10×10-3 |
|
|
9.67×10-4 |
5×10-5 |
5.0×10-3 |
|
|
1.29×10-3 |
5×10-5 |
5.78×10-3 |
|
|
1.92×10-3 |
5×10-5 |
6.50×10-3 |
|
|
3.22×10-4 |
1×10-5 |
1.47×10-3 |
|
|
3.22×10-4 |
2×10-5 |
1.56×10-3 |
|
|
3.22×10-4 |
3×10-5 |
1.63×10-3 |
|
|
3.22×10-4 |
4×10-5 |
1.63×10-3 |
|
|
3.22×10-4 |
5×10-5 |
1.50×10-3 |
2.2 Choice of analytical concentration scopes
From the standard stock solution of Zidovudine, suited aliquots were pipetted out into 10 milliliters volumetric flasks and dilutions were made with distilled H2O to obtain operational standard solutions of concentrations from 2 to 50 ?g / milliliter. Optical density for these solutions were measured at 266 nanometers and the spectra was shown in Figure 3. The most of legion pharmaceuticals compounds present in the scope of mcg per litre in H2O so in order to make up one’s mind the concentration the scopes are fixed in analytical scope. The concentrations of the criterions must lie within the working scope of the technique ( instrumentality )[ 10 ].
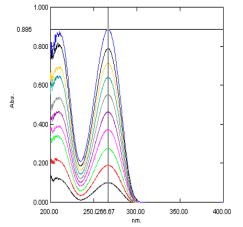
Fig 2.2:UV Spectra of Zidovudine at 266 nanometers[ 10 ]
2.3 Calibration Curve for the Zidovudine
Correct volumes of aliquots from standard Zidovudine stock solution were transferred to different volumetric flasks of 10 milliliter capacity. The volume was used to the grade with distilled H2O to obtain concentrations of 0.5, 1, 2, 2.5,3,4 and 5 ?g / milliliter. Absorbance spectra of each solution against distilled H2O as space were measured at 266 nanometers and the graphs of optical density against concentration were plotted and shown in Figure 4. The arrested development equation is determined.
Table 2.3: Consequences of standardization curve at 266nm for Zidovudine by Visible Spectroscopy
|
Sl.no |
Concentration ( µg/ml ) |
Optical density at 266nm |
|
1 |
1 |
0.146 |
|
2 |
2 |
0.264 |
|
3 |
4 |
0.482 |
|
4 |
5 |
0.499 |
|
5 |
6 |
0.653 |
|
6 |
7 |
0.865 |

Figure 2.3: One-dimensionality secret plan or standardization remedies for Zidovudine at 266 nanometers by Visible Spectroscopy.
Table 2.4: Optical Features of Zidovudine
|
Parameters |
Consequence |
|
Absorption upper limit |
266 nanometer |
|
Beer’s jurisprudence bound ( ?g / milliliter ) |
2-20 ?g / milliliter |
|
Correlation coefficient ( R2) |
0.997 |
|
Regression equation ( y = maxwell + degree Celsius ) |
Y = 10116x + 0.057 |
3 RESULTS AND DISCUSSION
The Analyses on rate of debasement of Zidovudine with mention to assorted parametric quantities were conducted and the consequences are tabulated, graphs plotted and in depth treatment are carried out as follows:
- Degradation surveies of Zidovudine withfluctuation of Drug.
Concentration of drug [ ZDV ] was assorted from 1×10-5M to 6 x10-5M for pH ( 3 ) and these two concentration are [ HOCl ] =3×10-4M, [ BUFFER ] = 6×10-3M. By following Beer Lambert Law graphs are plotted absorbance versus clip. The inside informations are enumerated in wining pages.

Fig 3.1: Graph demoing debasement of Zidovudine with regard to clip
Consequence of fluctuation of Zidovudine concentration of [ 1×10-5] M to [ 5×10-5] M with regard to clip shows debasements at peculiar concentration.The consequences are represented in above graph. The debasement rate is non much fast. Maximal debasement are at concentration of ZDV= [ 5×10-5] in pH 3. Zidovudine chemical reacts really less to chlorination procedure.
- Variation of HOCl
Concentration of HOCl is varied from 2.25×10-3to 3.22×10-4and staying are kept changeless for pH=3 and these two concentration are [ ZDV ] =5×10-5, [ BUFFER ] = 6×10-3. By following Beer Lambert Law graphs are plotted absorbance versus clip.

Fig 3.2: Graph demoing the debasement at pH = 3 at assorted concentration of [ HOCl ]
Consequence of fluctuation of HOCl concentration of [ 2.25×10-3] M to [ 3.22×10-4] M with regard k’obs shows debasements at peculiar concentration.The consequences are represented in above graph.The plotting points lie on a consecutive line bespeaking linarity. k”app=1.496 M-1Second-1is the pH dependent evident 2nd order rate.
- Variation of Dielectric Constant.
The consequence of dielectric invariable ( D ) was studied by changing the t- butyl alcohol H2O content in the reaction mixture with all other conditions are maintained changeless like [ ZDV ] =5×10-5[ HOCl ] =3×10-3, [ BUFFER ] =2×10-3

Fig 3.3: Consequence of dielectric invariable on the chlorination of Retrovir at 250C.Ionic strength = 0.02 moldiabetes mellitus-3at 3 pH
The consequence of dielectric invariable ( D ) was studied by changing the t-butanol H2O content in the reaction mixture with all other conditions being maintained changeless. The dissolver did non respond with the oxidant under experimental conditions. The rate changeless k’obs decreased with lessening in the dielectric invariable of the medium. The secret plan of log k’obs versus 1/D with points was additive with a negative incline and R2-0:939.
3.4 Chemical reaction Order
The concentration of ZDV was varied in the scope 1×10-5mol diabetes mellitus-3to 2×10-5mol diabetes mellitus-3and one-dimensionality of secret plans of log [ AT-A?] Vs clip ( r?0.9994 ) and indicated a reaction order of integrity in ZDV. A secret plan of log k’obs versus log [ HOCl ]Owith 5 points was additive with a incline of 0.968 and R2-0:992, bespeaking that this reaction can be treated as first order with regard to ZDV.The concentration of HOCl was varied in the scope 2.25 x10-3mol diabetes mellitus-3to 3.22×10-4mol diabetes mellitus-3and the secret plan of log KObVs log [ ZDV ] was found to be additive with incline near to integrity bespeaking unit order in HOCL.The reaction of ZDV with HOCl can be described as a bimolecular, 2nd order reaction.
ZDV + HOCL > Product
3.5 Effect of pH on The Chemical reaction
The pH of the reaction mixture was varied from pH=3 – 8.8 by utilizing ethanoate, phosphate and borate buffers, maintaining other conditions constant throughout the experiment. The rate of debasement was found to diminish with addition in pH.
3.6 Effect of ab initio added merchandises
Initially added merchandises such as chloramines or combined Cl ( CC ) do non hold any important consequence on the rate of reaction.
4 Decision
- Zidovudine reacts easy with HOCl at oxidant concentration and pH conditions, which are similar to those likely to be observed in conventional H2O chlorination processes.
- From intervention position, pharmaceuticals are non unusual organic chemicals, and their intervention remotion rates depend on the physical and chemical belongingss of the drug composing.
- Conventional intervention processes with chlorination ( free Cl ) can take approximately 50 % of these compounds, whereas advanced intervention procedures, such as ozonation, advanced oxidization, activated C and membranes ( e.g. rearward osmosis, nanofiltration ) , may be recommended to accomplish higher remotion rates, Reverse osmosis, for illustration, can take more than 99 % of big pharmaceutical molecules.
- The rate of debasement of Zidovudine decreases quickly above pH 3.Hence conventional Chlorination can non be applied for the remotion of Zidovudine.
- Consequence of Dielectric Constants on the rate invariables of Zidovudine and HOCl reaction indicates the reaction involves dipoles and charged species.
- Consequence of fluctuation of ionic strength on the rate of reaction indicates the reaction is either between two impersonal species or impersonal and a charged species.
- Limited informations on the happening of pharmaceuticals in drinking-water proved to be a large challenge in measuring possible human wellness hazards from exposure to follow concentrations of pharmaceuticals in drinking-water.
Mentions
- APHA ( American Public Health Association ) , AWWA ( American Water Works Association ) .and WPCF ( Water Pollution Control Facility ) , ( 1998 ) Standard methods for the scrutiny of H2O and effluent, 20th Edition. Washington DC, USA.
- Basavaiah.K. and Anil Kumar. UR., “Simple Spectrophotometric methods for the finding of Zidovudine in pharmaceuticals utilizing Chloramine-T, Methylene Blue and Rhodamine-B reagents”,E-Journal of Chemistry. 3 ( 12 )2006, pp 173-181.
- Cimons, Marlene. U.S. Approves Sale of AZT to AIDS Patients. Los Angeles1987Timess: p. 1.
- Dodd, M.C. and Huang, C.H.( 2004 ) Environ. Sci. Techn. , 38, 5607.
- Gouthami K, Anusha Priyadarshini K, Nikhila Soundarya A, Pushpa Latha E,“Spectrophotometric Method For Degradation Study Of Lamivudine” ,Department of Pharmaceutical Analysis and Quality Assurance, Creative Educational Society’s College of Pharmacy, Chinnatekur, Kurnool – 518218, Andhra Pradesh, India. Vol 3 / Issue 2 / 2013 / 66-69.
- Le-Minh.N, Khan.S.J, Drewes.J.E, Stuetz.R.M,“Destiny of antibiotics during municipal H2O recycling intervention procedures” , Water research 44 ( 2010 ) , pp ( 4296 ) .
- Mahadev S. Gudaganatti, Manjunath S. Hanagadakar, Raviraj M. Kulkarni, Ramesh S. Malladi and Rajaram K. Nagarale, “Transformation of levofloxacin during H2O chlorination procedure: dynamicss and tracts” , Progress in Reaction Kinetics and Mechanism. Vol. 37, pp ( 367-368 )
- Marie Deborde, Urs von Gunten,“Chemical reactions of Cl with inorganic and organic compounds during H2O treatment—Kinetics and mechanisms: A critical reappraisal” , Water Research 42, ( 2008 ) , pp ( 14 ) .
- Raviraj M Kulkarni, Manjunath S Hangadakar, Ramesh S Malladi, Mahadev S Gudaganatti, Himansu S Biswal, Sharnappa T Nandibewoor,“Transformation of linezolid during H2O intervention with Cl – A kinectic survey” , Indian Journel of Chemical Technology, Vol 21, January 2014, pp. ( 38-43 )
- Sharada.C. H, Channabasavaraj. K.P and Tamizh.T, Mani, “Development of a Spectrophotometric Method for the Quantitative Estimation of Zidovudine Concentration in Bulk and Pharmaceutical Dosage Forms” , Department of Pharmaceutical Analysis, Bharathi College of Pharmacy, Bharathi Nagara, Mandya District, Karnataka, India – 571 422. KMITL Sci. Tech. J. Vol. 10 No. 1 Jan. – Jun. 2010
- United States Pharmacopoeia ( USP-NF XXIV ) , Rockville MD 20852. United States Pharmacopoeial Convention Inc, 1985. p. 3489.
- World Health Organization International Agency For Research On Cancer, “IARC Monographs On The Evaluation Of Carcinogenic Risks To Humans” , vol 76, 2000, pp ( 73-127
- hypertext transfer protocol: //en.wikipedia.org/wiki/Zidovudine
- hypertext transfer protocol: //www.rxlist.com/retrovir-drug.htm
